Poisons: The Perfect Murder Weapon with DP Lyle, MD
MWA Rocky Mountain Chapter Event on YouTube
Many thanks to the MWA Rocky Mountain Chapter for hosting this event and asking me to do it.
Great group.
Poisons: The Perfect Murder Weapon with DP Lyle, MD
MWA Rocky Mountain Chapter Event on YouTube
Many thanks to the MWA Rocky Mountain Chapter for hosting this event and asking me to do it.
Great group.
Criminal Mischief: Episode #34: Toxicology Part 3
LISTEN: https://soundcloud.com/authorsontheair/episode-34-toxicology-part-3
PAST SHOWS: http://www.dplylemd.com/criminal-mischief.html
SHOW NOTES:
From HOWDUNNIT: FORENSICS
COMMON DRUGS, POISONS, AND TOXINS
In the remote past, most poisoners favored botanical products such as hemlock, oleander, deadly nightshade, foxglove, hellebore, monkshood, opium, and many others. These were easily available and untraceable. More recently, various chemicals have been added to this long list of plant-based poisons, which has made the work of the toxicologist that much more difficult.
I said earlier that when the forensic toxicologist is faced with determining whether an individual’s death or abnormal behavior is related to toxin exposure, he will use all evidence, including the results of toxicology testing, the autopsy examination, and statements from investigating officers and witnesses. To effectively use this information, he must be familiar with many aspects of drugs and poisons: He must know the chemical makeup and physiological actions of drugs and their breakdown products; understand how drugs are metabolized in the body and what the potential toxic properties of these metabolites are; know how these chemicals affect a normal person, as well as those with various illnesses and addictions; and be aware of the symptoms and signs produced by these chemicals. In addition, a working knowledge of street and recreational drugs is essential, since these are often involved in injury and death.
Obviously, a discussion of every known chemical, drug, and poison is far beyond the scope of this book. We will, however, look at many of those that crop up in real-life cases as well as in works of fiction.
We will examine the things that the ME and the toxicologist consider in assessing the effects of any of them in death, injury, or legal matters. For example, some drugs cause severe depression or addiction and may lead the user to take his own life. Others may distort perceptions to the point that the user accidentally causes self-harm through some foolish act. Trying to fly from a building would fit this description. Other drugs may cause anger, aggression, or an actual psychotic episode, and the user may commit assaults or homicides while under the chemical’s influence. Some drugs are so addictive that the user will commit all types of illegal acts (robbery, assault, or murder) to obtain money to purchase them. Other substances are just downright deadly.
ALCOHOL
Alcohol is derived from the fermentation of sugars and comes in a variety of types, with ethanol (ethyl, or drinking alcohol), methanol (methyl, or wood or denatured alcohol), and isopropanol (isopropyl, or rubbing alcohol) being the ones most commonly encountered. All alcohols are central nervous system (CNS) depressants. Central nervous system basically means the brain. These alcohols cause sleepiness, poor coordination, slowed movements and reactions, and distorted perceptions. In short, all the symptoms and signs you recognize in someone who is drunk. In larger amounts, they can lead to coma, cessation of breathing, and death from asphyxia.
Ethanol
Ethanol is by far the most commonly abused drug. Not only are its toxic effects potentially lethal, but the loss of coordination and poor judgment that is associated with its use can lead to violent and negligent acts. There is potential for physical addiction with alcohol and withdrawal can be an arduous and dangerous process. Without proper medical treatment, death rates from alcohol withdrawal syndromes, such as delirium tremens (DTs), can be 20 percent or more.
Alcohol in the body follows a fairly simple pathway. Once ingested, it is absorbed into the bloodstream and disseminated throughout the body, where 95 percent of it is metabolized (broken down) by the liver into water and carbon dioxide. The remaining 5 percent is excreted unchanged through the kidneys and lungs, a fact that is critical to sobriety testing.
Alcohol Metabolism
The body eliminates most toxins in what is called a dose-dependent fashion; that is, the higher the dose taken, the more rapidly the toxin is metabolized. A small amount activates only some of the enzymes that break down the toxin, whereas a larger amount activates more enzymes in order to handle the increased load of toxin.
Alcohol is metabolized in a linear fashion in that any amount of alcohol intake activates all the enzyme systems that destroy it. This means that from the first drink, the system operates at almost maximum efficiency and there is little or no ability to increase it. The average rate of ethanol destruction in the body is roughly equivalent to one drink per hour.
Why is this important? With rapid intake of alcohol, as is seen in binge drinking that is so common among college students, the body has no method for increasing the removal of the alcohol. The system is already running at top speed and excessive intake overruns the body’s ability to deal with it. The result is that the concentration of alcohol in the blood will rise rapidly and this can lead to coma and death.
Methanol
All alcohols are potentially toxic, but methanol is particularly so. Methanol is the denatured alcohol used in Bunsen burners in high school science or chemistry classes. Unlike ethanol, the liver converts methanol to formic acid and formaldehyde, the same stuff the coroner uses to preserve the tissues he removes from corpses.
Methanol ingestion causes nausea, vomiting, pancreatic and other organ damage, confusion, loss of coordination, and brain damage that can lead to blindness, seizures, coma, and ultimately death from asphyxia.
Isopropanol
Isopropanol is also an intoxicant and a CNS depressant whose effects usually appear within ten to thirty minutes after ingestion, depending upon the amount consumed and whether food or other beverages are taken as well. Fifteen to 20 percent of ingested isopropanol is converted to acetone, which produces acidosis (excess acid in the body). This greatly complicates things. The victim appears drowsy and off balance, and possesses a staggering gait, slurred speech, and poor coordination. Nausea, vomiting (sometimes bloody), abdominal pain, sweating, stupor, coma, and death from respiratory depression may follow. Hemorrhage into the bronchial tubes (breathing tubes or airways) and chest cavity may occur.
Isopropanol also absorbs through the lungs and the skin. Not infrequently, infants experience isopropanol toxicity from alcohol-and-water sponge baths used to treat childhood fevers.
OTHER CNS DEPRESSANTS
Opiates, barbiturates, and other tranquilizers are CNS depressants. They make a person sleepy and lethargic and are called downers.
Opiates
Opiates are in the alkaloid family of chemicals and are derived from the sap of the poppy. The opiates are divided into natural, semisynthetic, and synthetic, depending upon their source and method of manufacture. They are narcotic sedatives (sleep producing) and analgesics (pain relieving) that produce euphoria, lethargy, and, in larger doses, coma and death from respiratory depression and asphyxia. This is more common when an opiate is mixed with alcohol, which is also a brain depressant. Most opiates are taken either by mouth or injection, and all have great potential for abuse and physical addiction.
Natural opiates come directly from the poppy with morphine, a powerful narcotic much like heroin, and codeine being the basic ones. Codeine is found in many cough suppressants, has a low potential for abuse, and, unless used with alcohol, rarely causes death. Combining morphine with acetic anhydride or acetyl chloride produces heroin (diacetylmorphine), which is by far the most commonly abused opiate.
After injection, heroin is almost immediately broken down into monoacetylmorphine and then to morphine. In the living user, testing typically only reveals morphine since this two-step conversion process occurs fairly quickly. This means that the testing cannot determine if the person used heroin or morphine, since in either case only morphine would be found.
The autopsy findings in individuals who die from a heroin overdose are fairly consistent. The ME usually, but not always, finds evidence of pulmonary edema, which is water in the lungs. The lungs often show evidence of talc crystals and cotton fibers, as these are used to cut and filter the heroin, respectively. When the drug is given intravenously, these crystals and fibers are carried through the right side of the heart and are filtered from the blood and trapped by the lungs.
Semisynthetic opiates are created by molecular alterations of morphine and codeine. Many medical analgesics are of this type. Hydrocodone, oxymorphone, and oxycodone (OxyContin) are examples.
Synthetic opiates are constructed in a laboratory and are not derived from either morphine or codeine. Methadone is the best known of this class because of its use in treating heroin addiction. Other synthetic opiates include meperidine (Demerol) and fentanyl drugs.
Barbiturates
Barbiturates are derived from barbituric acid. Known as hypnotics (sleeping pills), they include pentobarbital, amobarbital, secobarbital, butabarbital, and phenobarbital. Only phenobarbital, an excellent anticonvulsive (prevents seizures) medication, is widely used today. When mixed with alcohol, barbiturates can readily lead to coma and death from asphyxia.
CNS STIMULANTS
Stimulants or “uppers” are a commonly abused class of drugs that rev up the nervous system and pump up the blood pressure and heart rate. The ones most commonly used are amphetamines and cocaine. These drugs increase alertness, lessen fatigue, and suppress appetite. However, with continued use, they cause irritability, anxiousness, aggressive behavior, paranoia, fatigue, depression, and death.
Chronic users tend to develop tachyphylaxis. This means that the body “gets used to” them and their effects are lessened. The user must take ever-increasing amounts to get the same “kick” because the body produces more of the enzymes that metabolize these drugs so that they are destroyed and eliminated at a faster rate.
Amphetamines
Amphetamines belong to the phenethylamine class of chemicals and are what we term sympathomimetics, in that they mimic, or act like, the sympathetic side of the autonomic nervous system. This is the fight or flight response. Amphetamines rev up the body for emergency action. To do this, they increase blood pressure, heart rate, and respiration, and produce euphoria and a sense of high energy.
Cocaine
Cocaine is a CNS stimulant that increases alertness, elevates blood pressure and heart rate, and raises body temperature. In higher amounts, it can lead to seizures, strokes, heart attacks, and death.
Typically, cocaine is snorted, or inhaled through the nose. When introduced this way, it rapidly absorbs through the membranes that line the nose and enters the bloodstream. Its effects are felt in just a few minutes. As with amphetamines, cocaine has the problem of tachyphylaxis so the “high” tends to diminish with repeated use. So, abusers have found even faster ways of reaching the “high” they seek.
Cocaine can be mixed with baking soda and water, and heated until all the liquid is evaporated; the solid material remaining is crack cocaine. This form has a much lower boiling point (becomes a gas at a lower temperature), which allows it to be smoked. When inhaled, this gaseous form is very rapidly absorbed through the lungs and into the bloodstream.
HALLUCINOGENIC DRUGS
Hallucinogens alter perceptions and mood, lead to delusional thinking, and cause hallucinations.
Delusions are beliefs that have little or no basis in reality. The person might believe that he is being watched or monitored or that his neighbor, boss, or spouse is trying to harm him.
Hallucinations are sensory experiences that are not real. That is, they are not an abnormal sensing of some sensory input; rather, the entire sensory experience is created within the person’s mind. These creations may involve any or all of the senses. They may be visual, auditory, olfactory, taste, or tactile. Sometimes these sensations are so real that the person can’t separate the hallucination from reality, or worse, the hallucination becomes the reality.
Hallucinations are part of severe schizophrenia and other mental disorders and can occur in victims of strokes or senile dementia. They are often seen with use and withdrawal from alcohol and other drugs. Hallucinogenic drugs are specifically designed to produce hallucinations.
The most frequently encountered hallucinogens come from the plant world (marijuana, peyote, and mushrooms) or the chemistry laboratory (LSD, STP, and PCP). Their identification depends upon both physical and chemical analyses.
Cannabinoids
By far the most commonly used hallucinogen, and one of the mildest, is marijuana. It goes by many street names including Mary Jane, weed, and pot. It is a cannabinoid, which means it is derived from the Cannabis sativa plant. The active ingredient tetrahydrocannabinol (THC) is found in marijuana at a concentration of 2 to 6 percent. Hashish is the oily extract of the plant and contains approximately 12 percent THC.
Though marijuana can be added to food and eaten, the most common method of introduction is through smoking. It is rapidly absorbed through the lungs, reaches peak blood levels in fifteen to twenty minutes, and usually lasts about two hours. It produces euphoria, sedation, loss of memory, reduced coordination, and also stimulates appetite.
The body breaks down THC into a series of compounds, the most important being 9-carboxy-tetrahydrocannabinol (9-carboxy-THC), which is the major urinary metabolite. Urine drug testing looks for this compound, which can be found up to ten months after last use. One problem is that even passive exposure can lead to a positive urine test. For example, if a person is in the area where someone is smoking marijuana, his urine may reveal low levels of 9-carboxy-THC.
Cacti and Mushrooms
Peyote is a small Mexican cactus that has enjoyed a ceremonial use by many native tribes for centuries. The active chemical in the plant is mescaline, which is a hallucinogen in the alkaloid family. Either TLC or GC can confirm the presence of the alkaloids. Further testing to identify mescaline is not necessary since the possession of plant material itself is illegal.
Mushrooms present a different problem. With marijuana and peyote the mere possession of the plant is illegal, while the possession of mushrooms is not. This means that the toxicology lab must identify the psychoactive components (psilcin and psilocybin) of the mushroom before they can be deemed illegal.
LSD AND OTHER HALLUCINOGENIC CHEMICALS
There are a wide variety of chemically produced hallucinogens, with the most common ones being lysergic acid diethylamide (LSD) and phencyclidine (PCP or angel dust).
LSD is very potent and as little as 25 micrograms can produce an “acid trip” that lasts for twelve hours. Though LSD is not directly fatal, the hallucinations it produces are typically vivid and there have been many instances of users harming themselves because of these altered perceptions. The primary screening test for LSD is the Van Urk color test.
PCP is an extremely powerful drug with unpredictable effects. It comes as a powder or in a capsule or pill. It can be swallowed or smoked. PCP can cause depression, irritability, feelings of isolation, and is notorious for producing psychosis, paranoia, and violent behavior. An acute schizophrenic episode may suddenly occur many days after use. In a large enough dose, it can cause seizures and death.
Immunoassay of urine is used for PCP screening and may remain positive for a week after last use. GC/MS provide confirmation.
Other chemical hallucinogens include dimethoxymethylamphetamine (STP), dimethyltryptamine (DMT), and methylenedioxymethamphetamine (MDMA), also known as ecstasy.
DATE RAPE DRUGS
The date rape drugs are a collection of chemicals of various types that share the ability to make the user relaxed, disoriented, and compliant. Some are pharmaceutically manufactured, while others are cooked up by someone with marginal experience and a chemistry book. The major members of this group are Rohypnol (flunitrazepam), ecstasy, GHB (gamma-hydroxybutyrate), and ketamine hydrochloride.
Rohypnol, GHB, and ketamine are commonly used in date or acquaintance rapes, which is where the moniker comes from. They cause sedation, a degree of compliance, poor judgment, and amnesia for events that occur while under their influence. These properties make them effective in date rape situations.
A small amount of GHB or Rohypnol can be slipped into the victim’s drink or a bottle of innocuous-appearing water. She may appear and act normally, or might seem happy, excited, pleasantly sedated, or mildly intoxicated. Neither the victim nor her friends recognize how impaired she actually is. She might leave with her would-be assailant because her judgment is impaired and euphoria enhanced. Only later will she realize that something happened, but her memory of events will be spotty or absent. This is exactly what happened with Andrew Luster’s victims.
The reactions to these drugs are unpredictable and vary from person to person.
Rohypnol (Street Names: Roofies, Roaches, Rope, Mexican Valium) is a benzodiazepine sedative in the same family as Valium and was developed to treat insomnia. Currently, it is neither manufactured nor approved for use in the United States, but is available in Mexico and many other countries. It is manufactured as white tablets of either one or two milligrams that can be crushed and dissolved in any liquid. It takes action twenty to thirty minutes after ingestion, peaks in about two hours, and its effects may persist for eight to twelve hours.
Rohypnol typically causes sedation, confusion, euphoria, loss of identity, dizziness, blurred vision, slowed psychomotor performance, and amnesia. The victim has poor judgment, a feeling of sedated euphoria, and vague or no memory of what has happened.
Ecstasy (Street Names: E, X, XTC, MDMA, Love, Adam) was first manufactured in 1912 and originally patented in 1914 as an appetite suppressant but was never marketed. It disappeared until the 1960s when it was rediscovered and became a drug of abuse. Currently, it is made in underground labs and distributed in pill or capsule form. It has amphetamine (speed-like) as well as hallucinogenic effects. The user has enhanced sensations and feelings of empathy, increased energy, and occasionally profound spiritual experiences or irrational fear reactions. It may cause increased blood pressure, teeth grinding (bruxia), sweating, nausea, anxiety, or panic attacks.
GHB comes as a white powder that easily dissolves in water, alcohol, and other liquids. Currently, it is often found as “Liquid E,” a colorless, odorless liquid that is sold in small vials and bottles.
The effects of GHB appear five to twenty minutes after ingestion and typically last for two to three hours. It causes loss of inhibitions, euphoria, drowsiness, and, when combined with other drugs, increases the effects of these drugs. Users might also experience amnesia, enhanced sensuality, hallucinations, and amnesia.
Ketamine is a rapidly acting intravenous or intramuscular anesthetic agent that causes sedation and amnesia. It comes as a liquid, which is often heated and evaporated to a white powder residue. The powder can be added to a liquid such as a bottle of water, compacted into pills, or, most commonly, snorted. Whether swallowed or snorted, it takes effect almost immediately and is fairly short in its duration of action, typically forty-five minutes to two hours.
Many of its effects are similar to ecstasy, but it also possesses dissociative effects, which means the person “dissociates” from reality in some fashion. Often the user experiences hallucinations, loss of time sense, and loss of self-identity. One common form is a depersonalization syndrome, where the person is part of the activities while at the same time is off to the side or hovering overhead watching the activity, including his own actions. As mentioned earlier, this reaction is also common with PCP. Users call these effects “going into a K Hole.”
Since ketamine is a sedative and general anesthetic, its potential for serious and lethal effects is real. If too much is taken, the victim may lose consciousness, stop breathing, and suffer brain damage or die.
MISCELLANEOUS TOXINS
Cyanide is one of the most lethal chemicals known and can enter the body by inhalation, ingestion, or directly through the skin. The most common forms are the white powders sodium cyanide (NaCN) and potassium cyanide (KCN) and the gaseous hydrogen cyanide (HCN). Most poisonings are accidental, but suicidal and homicidal cyanide poisonings do occur. HCN is used in gas chamber executions. Cyanide is a metabolic poison, which means it damages the internal workings of the cells.
Strychnine is a neuromuscular toxin that causes powerful convulsive contractions of all the body’s muscles. The body adopts a posture known as opisthotonos, which means the back is arched so that only the back of the head and the heels of the feet touch the floor. Death results from asphyxia, since breathing is impossible during such violent muscular contractions. At death, rigor mortis often occurs very quickly because the muscles are depleted of ATP during these contractions (see Chapter Five: Time of Death, “Rigor Mortis”). Strychnine is rarely used for homicide since its extremely bitter taste makes it difficult to disguise in food. It is occasionally used for suicide, but since it has the deserved reputation for being very painful, this is also rare.
Mushrooms were discussed earlier (see “Hallucinogenic Drugs”). But those of the psilocybin variety are not nearly as sinister as are the mushrooms of the Amanita family, such as the death cap and death angel mushrooms. These poisonous mushrooms have been implicated in accidental, suicidal, and homicidal deaths.
The death cap is so toxic that a single mushroom can kill. The two main toxins are amanitin, which causes a drop in blood sugar (hypoglycemia), and phalloidin, which damages the kidneys, liver, and heart. The real treachery of these mushrooms lies in that fact that the symptoms—nausea, vomiting, diarrhea, and abdominal pain—are slow to onset, typically beginning six to fifteen hours after ingestion, but can be delayed as much as forty-eight hours. In general, the later the onset of symptoms, the worse the chances for survival. This is because the toxins go to work on the liver and other organs almost immediately, but since symptoms are delayed for many hours, the victim doesn’t know to seek medical help until it is very late. At autopsy, the ME finds severe damage to the liver and the toxicologist might find a low level of sugar in the blood, as well as the amantin and phalloidin toxins.
Ethylene glycol is the major ingredient in many antifreeze solutions. In the body, ethylene glycol breaks down into several compounds, the most important being oxalic acid. When oxalic acid is absorbed into the bloodstream, it reacts with calcium in the blood to form calcium oxalate. This reaction consumes the blood’s calcium, and low levels can cause a cardiac arrest and death. The calcium oxalate is filtered through the kidneys where it can clog up the microscopic tubules and severely damage the kidneys. At autopsy, the ME finds the crystals in the tubules of the kidney. Oxalic acid is also found in raw (not properly cooked) rhubarb, which can lead to accidental poisonings. It is rarely if ever used for suicide or homicide. Ingestion of this plant can irritate the gastrointestinal tract and cause mouth, throat, and esophageal pain and possibly bleeding. In those who die from this plant, the autopsy reveals a burned and irritated mouth, esophagus, and stomach, low blood calcium levels, and calcium oxalate sludge in the kidneys.
Heavy metals are dangerous metallic elements such as arsenic, mercury, lead, bismuth, antimony, and thallium. Arsenic was the major homicidal poison for hundreds of years but is not frequently used now. One reason is that it works slowly. Even a large dose will take hours to kill someone. And since the death is very painful, the victim will often seek medical help before death and sur- vive. It is occasionally used as a chronic poison.
The most common arsenical compound used in homicidal poisonings is arsenic trioxide, which is a white powder. A dose of 200 to 300 milligrams is usually lethal. Symptoms begin about thirty minutes after ingestion and include nausea, vomiting, abdominal pain, bloody diarrhea, a metallic taste in the mouth, and a slight garlicky odor to the breath. Arsenic severely damages the lining of the stomach and intestines and the ME will easily see this at autopsy in those who die. He will also find fatty deposits in the liver, kidneys, and heart.
Lead poisoning is uncommon and usually occurs in an industrial setting. Occasionally children will peel away and eat wall paint that contains lead. Even though lead has not been a component of interior paints for decades, many older buildings still have layers of old lead-based paint. Lead poisoning can cause anemia, nausea, vomiting, abdominal pain, weakness, numbness, and seizures.
Insulin is a naturally occurring hormone that is essential for life. It is also synthetically manufactured and is a life-saving treatment for many diabetics. On occasion, diabetics die from an accidental overdose of insulin, but it has also been used for suicide and homicide. In fact, for many years it was considered to be an almost perfect murder weapon. The injection of a large dose dramatically drops the level of sugar in the blood, and since the brain needs a continuous supply of nutrition, death occurs very quickly. And since insulin is normally found in all of us, how could its presence raise suspicion? Now, insulin levels can be determined by radioimmunoassay and if the level at autopsy is found to be very high, the ME searches for a rare insulin-secreting tumor in the pancreas. If he finds no tumor, it is logical to suspect that insulin has been administered by someone else and the ME launches a search for hidden injection sites on the corpse.
Succinyl choline is an injectable drug that paralyzes every muscle of the body and prevents all movement, even breathing. Death is from asphyxia. It has also been considered a nearly perfect murder weapon.
After injection, it is very quickly metabolized by the body and leaves behind little evidence of its presence. However, since the 1980s the gas chromatography and mass spectrometry (GC/MS) combination has allowed for detection of the drug’s metabolites. If the ME suspects that this drug has been used, he takes blood samples as well as excises the tissues around any suspected injection sites and sends these materials to the toxicologist. Toxicological testing using GC/MS is directed toward finding metabolites of the drug, which, when found, proves that the drug was at one time present in the victim. This sophisticated testing was a direct result of the Carl Coppolino case.
To dig deeper into this subject grab a copy of either
FORENSICS FOR DUMMIES: http://www.dplylemd.com/book-details/forensics-for-dummies.html
HOWDUNNIT: FORENSICS: http://www.dplylemd.com/book-details/howdunnit-forensics.html
Criminal Mischief: Episode #27: ABO Blood Typing
LISTEN: https://soundcloud.com/authorsontheair/27-abo-blood-typing
PAST SHOWS: http://www.dplylemd.com/criminal-mischief.html
SHOW NOTES: http://www.dplylemd.com/criminal-mischief-notes/27-abo-blood-typing.html
ABO Blood Type System
From FORENSICS FOR DUMMIES
By simply typing the blood at a crime scene, investigators narrow their suspect list and completely exonerate some suspects by using the population distribution information for the four ABO blood types.
Population Distribution of ABO Blood Types
O: 43%
A: 42%
B: 12%
AB: 3%
Besides determining the ABO type, serologists are able to further individualize blood samples. RBCs contain more proteins, enzymes, and antigens than those used in the ABO classification system. These include antigens with such catchy names as Duffy, Kell, and Kidd and intracellular enzymes such as adenylate kinase, erythrocyte acid phosphatase, and the very useful phosphoglucomutase (PGM).
PGM is an enzyme that appears in many different forms, or isoenzymes, and at least ten of them are fairly common. Regardless of ABO type, a particular individual can have any combination of the isoenzymes of PGM. The ME and the serologist use that fact to further narrow the list of suspects for further DNA analyses and confirmation that they were capable of leaving a particular bloodstain.
For example, say that a stain is Type AB and has PGM 2. The ME knows the AB blood type is found in only 3 percent (see Table 14‐1) of the population, and PGM 2 is found in only 6 percent of people. Because these two factors are inherited independently, the probability of a particular individual being Type AB, PGM 2 is only 0.18 percent or less than 2 per 1,000.
If the police find blood at the scene that matches the blood of a suspect who has Type AB, PGM 2 blood, the probability that that suspect is not the perpetrator is 2 in 1,000. Although not perfect, those odds still are much better than a coin toss.
Testing for Paternity
You inherit your blood type from your parents. For that reason, a serologist can assess paternity in many cases. The crime lab is often involved in paternity testing because paternity may be a critical component in determining child support, custody, and visitation. It also may play an important role in crimes and civil proceedings that involve kidnappings, insurance fraud, and inheritance conflicts.
Inheriting your blood type
ABO blood types, or phenotypes, come in only four varieties: A, B, AB, and O. But, for some blood types two genotypes, or gene pairings, are possible. A phenotype is what something looks like (in this case the ABO blood type), while the genotype is the underlying genetic pattern. We receive our ABO genes from our parents, one from Dad and one from Mom.
The important thing to know in this system is that A and B genes are co-dominant (equally dominant), while the O gene is recessive. So someone who receives an A gene from one parent and an O gene from the other has Type A blood, but not Type O, because the A gene is dominant.
Determining Possible Genotypes from Phenotypes
Type A: AA or AO
Type B: BB or BO
Type AB: AB
Type O: OO
People with Type O blood must have an OO genotype. They can have neither an A nor a B gene because having one or the other dominates the O gene and produces either Type A or Type B blood.
A person with Type A blood can either receive an A gene from each parent and thus have an AA genotype or an A gene from one parent and an O gene from the other for an AO genotype. Remember, A is dominant, so when it is paired with the recessive O gene, the A gene determines blood type. People with the AA and AO genotypes both have Type A blood, but genetically speaking, they’re different.
Type A parents who have AA genotypes can provide only A genes to their offspring, because all their eggs or sperm have an A gene. But Type A parents who have AO genotypes can provide either an A gene or an O gene to their offspring, because half their eggs or sperm have an A gene, and the other half have an O gene. When both parents are Type A, several possibilities exist for the genotype their offspring will have.
In each of the scenarios presented in Figure 14‐1, the child’s blood type is Type A, except when both parents donate an O gene. In the latter case, the child’s genotype and blood type (phenotype) respectively are OO and Type O. These parents can’t have any offspring who have Type B phenotype or BB, BO, or AB genotypes, because neither parent has a B gene to donate.
Determining Fatherhood
Blood typing can exclude paternity but cannot absolutely verify it. For example, a man with Type AB blood can’t father a child with Type O blood. So if a child has Type O blood, all men with the Type AB are ruled out as the child’s father. A man with Type A (genotypes AA or AO) blood can be the father, but only if he has an AO genotype. Men who have AA genotypes also are excluded. Men with the AO genotype, however, can’t be ruled out at this point.
To dig deeper into this complex system grab a copy of either:
FORENSICS FOR DUMMIES: http://www.dplylemd.com/book-details/forensics-for-dummies.html
HOWDUNNIT: FORENSICS: http://www.dplylemd.com/book-details/howdunnit-forensics.html
Criminal Mischief: Episode #07: Famous and Odd DNA Cases
LISTEN: https://soundcloud.com/authorsontheair/criminal-mischief-episode-07-famous-odd-dna-cases
PAST SHOWS: http://www.dplylemd.com/criminal-mischief.html
FAMOUS AND ODD DNA CASES NOTES:
Colin Pitchfork: The Beginning
http://aboutforensics.co.uk/colin-pitchfork/
Timothy Wilson Spencer, The Southside Strangler” First US DNA Conviction
(David Vasquez—first to be exonerated by DNA)
https://en.wikipedia.org/wiki/Timothy_Wilson_Spencer
http://www.digitaljournal.com/article/352011
Brown’s Chicken Murders:
https://en.wikipedia.org/wiki/Brown%27s_Chicken_massacre
https://chicago.cbslocal.com/2018/01/08/browns-chicken-massacre-25-years-anniversary/
Lonnie Franklin, The Grim Sleeper: Familial DNA
https://en.wikipedia.org/wiki/Grim_Sleeper
James Lynn Brown: Familial DNA
https://www.ocregister.com/2012/12/04/family-members-dna-solves-1978-killing/
Gary Ridgway, The Green River Killer
https://en.wikipedia.org/wiki/Gary_Ridgway
Pierre G: Kiss DNA Foils Jewel Thief
David Stoddard: Dog Bite DNA Case
Maggot DNA Case:
https://www.ncbi.nlm.nih.gov/pubmed/22971153
Willow Martin Arson Case and Potato DNA:
http://www.courant.com/breaking-news/hc-strippers-arson-drugs-0713-20160712-story.html
https://www.mycitizensnews.com/news/2018/05/woman-sentenced-to-8-years-for-arson/

To say that DNA had revolutionized criminal investigations would be a huge understatement. Prior to DNA profiling, identifying a suspect with absolute certainty was more difficult. Fingerprints would work, of course, and eyewitness accounts, though flawed in many ways, could also help. But a criminal leaving behind biological evidence such as blood, semen, saliva, hair, skin cells, and other little bits, offers a method of identity that is second to none. DNA profiling has been used to catch many a criminal. But, in order for it to do its work, there must be something for the DNA analyst to compare the crime scene sample against. The DNA database, CODIS, helps because it stores millions of DNA profiles and if the perpetrator is in the system, a match can be made. But if he is not, the database is of little help.
DNA analysis can reveal the gender of the person who left behind the sample quite easily. But our DNA controls more than that. It determines how tall we will be, what our hair and eye color will be, our intellectual level, our ability to play music, and many other things. Familial DNA has been used to narrow down unknown samples to a smaller group, such as an extended family. And lately, this is been used in conjunction with the various ancestral databases to solve some crimes. But a newer technique offers another tool on the DNA front. It’s called DNA Phenotyping.
The principle seems simple: Since our DNA determines what we look like, would it not be possible to take a DNA sample and then create an image of the individual it belonged to? Maybe. At least great strides have been made in that regard. A case in point is that of research biologist Le Bich-Thuy, who was raped, battered, and strangled 24 years ago. DNA obtained from that scene was subjected to DNA Phenotyping and an image of the individual who likely perpetrated the crime was generated. Not only that, the image was age altered so that it would more accurately reflect what he might look like now. Fascinating case.

NMR Spectrograph
GHB is one of the so-called Date Rape Drugs—along with Ecstasy, Rohypnol, and Ketamine. I have an article on these on my website (See Below).
GHB has been difficult to detect, primarily because it’s rapidly metabolized (destroyed) by the body. But new techniques employing Nuclear Magnetic Resonance (NMR) Spectroscopy allow the detection of GHB metabolites (breakdown products) as much as 24 hours later. This gives investigators a longer time period to uncover GHB in a victim.
GHB can also often be found in the victim’s hair up to a month or more after exposure, but this testing is not as yet perfected.
https://www.forensicmag.com/news/2017/08/chemists-discover-marker-date-rape-drug-testing
http://www.dplylemd.com/articles/date-rape-drugs.html
https://www.ncbi.nlm.nih.gov/pubmed/25433016
More on the fascinating world of Forensic Toxicology can be found in FORENSICS FOR DUMMIES:
http://www.dplylemd.com/book-details/forensics-for-dummies.html

There are no bigger names in the history and development of modern crime scene investigation than French investigator Edmond Locard and his Austrian counterpart Hans Gross. These two men shaped the development of crime scene investigation and even today their techniques create the cornerstone of forensic science. Locard’s Exchange Principle underlies every forensic technique.

EDMOND LOCARD

HANS GROSS
They were also great fans of Sir Arthur Conan Doyle’s Sherlock Holmes and R. Austin Freeman’s Dr. John Evelyn Thorndyke. Locard even suggested that students of police procedure read the Sherlock Holmes stories and learn from his techniques.
Both the real-life investigators and the fictional ones had one thing in common: the careful and meticulous approach to any crime scene, taking care to collect all useful evidence, while not damaging or contaminating it.
In my book Forensics For Dummies, the methods and techniques used to evaluate a crime scene and collect evidence are explained in great detail. Check it out if you want to know more about the techniques that saw their origin more than 100 years ago.

Arsenic has, over the centuries, garnered many colorful names. It was called the “queen of poisons” because it was so readily available, easy to use, highly effective, and untraceable. Thus, it was used by many famous historical poisoners. Some called it the “king of poisons” but since over the years, female killers have favored poisons, “queen” seems more apt. It was also called “inheritance powder,” for obvious reasons—-once the estate holder is dead and gone, the heirs can party down.
Arsenic is the nearly perfect poison. This was definitely true centuries ago when there was no way to trace it. But what about today, with modern toxicological techniques? Unfortunately, arsenic is still a pretty good choice for the poisoner. It’s not often looked for in unexplained deaths and its effects mimic many medical conditions, particularly neurological and gastrointestinal.
Back a couple of centuries ago, because of its common use, a method for finding arsenic in the dead or ill became an imperative. There were many steps along this path. This search for arsenic was essentially the beginning of forensic toxicology.
From HOWDUNNIT: FORENSICS
Arsenic had been a common poison for centuries, but there was no way to prove that arsenic was the culprit in a suspicious death. Scientists had to isolate and then identify arsenic trioxide—the most common toxic form of arsenic— in the human body before arsenic poisoning became a provable cause of death. The steps that led to a reliable test for arsenic are indicative of how many toxicological procedures developed.
1775: Swedish chemist Carl Wilhelm Scheele (1742–1786) showed that chlorine water would convert arsenic into arsenic acid. He then added metallic zinc and heated the mixture to release arsine gas. When this gas contacted a cold vessel, arsenic would collect on the vessel’s surface.
1787: Johann Metzger (1739–1805) showed that if arsenic were heated with char- coal, a shiny, black “arsenic mirror” would form on the charcoal’s surface.
1806: Valentine Rose discovered that arsenic could be uncovered in the human body. If the stomach contents of victims of arsenic poisoning are treated with potassium carbonate, calcium oxide, and nitric acid, arsenic trioxide results. This could then be tested and confirmed by Metzger’s test.
1813: French chemist Mathieu Joseph Bonaventure Orfila (1787–1853) devel- oped a method for isolating arsenic from dog tissues. He also published the first toxicological text, Traité des poisons (Treatise on Poison), which helped establish toxicology as a true science.
1821: Sevillas used similar techniques to find arsenic in the stomach and urine of individuals who had been poisoned. This is marked as the beginning of the field of forensic toxicology.
1836: Dr. Alfred Swaine Taylor (1806–1880) developed the first test for arsenic in human tissue. He taught chemistry at Grey’s Medical School in England and is credited with establishing the field of forensic toxicology as a medical specialty.
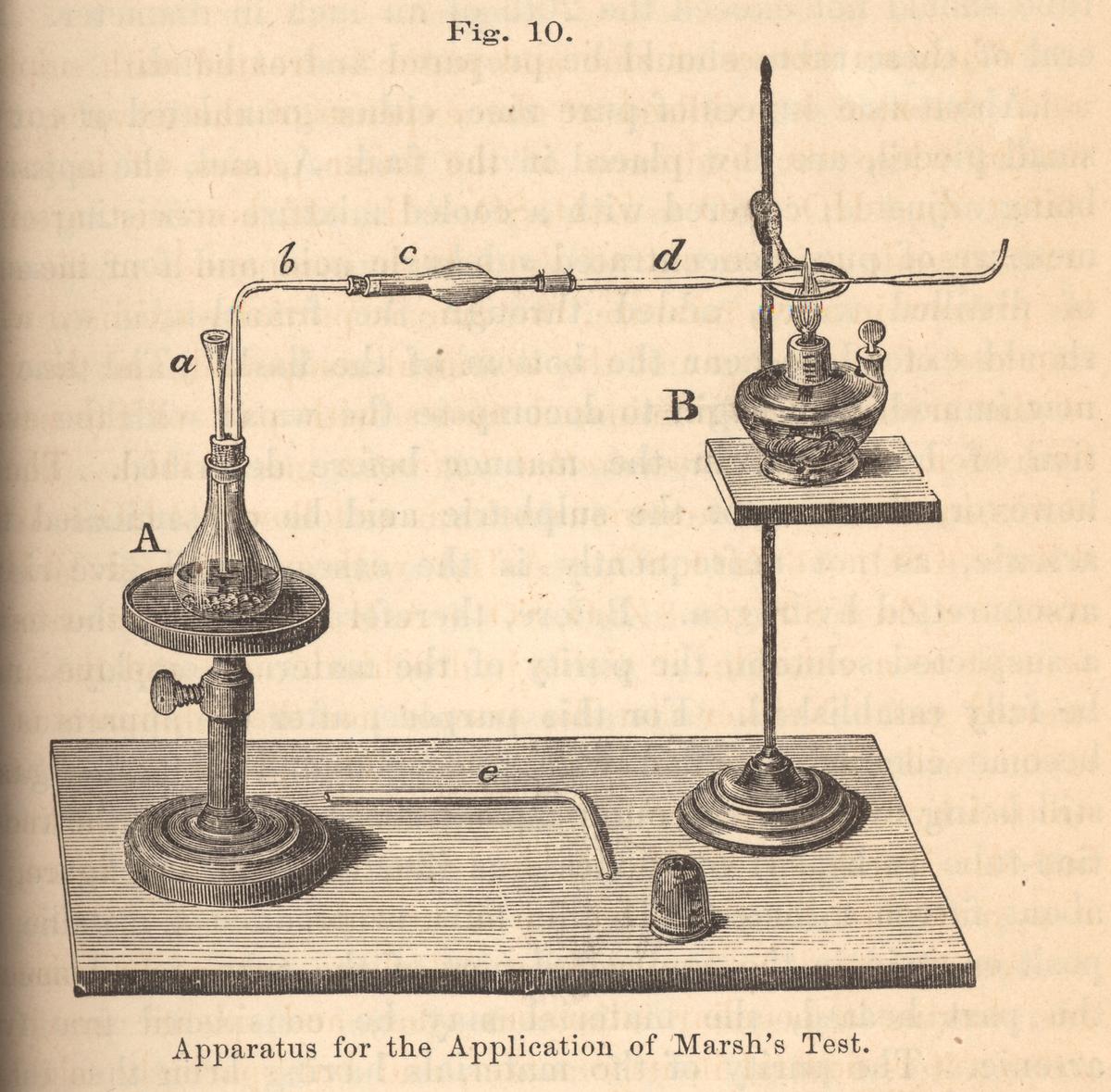
1836: James Marsh (1794–1846) developed an easier and more sensitive version of Metzger’s original test, in which the “arsenic mirror” was collected on a plate of glass or porcelain. The Marsh test became the standard, and its principles were the basis of the more modern method known as the Reinsch test, which we will look at later in this chapter.
As you can see, each step in developing a useful testing procedure for arsenic stands on what discoveries came before. That’s the way science works. Step by step, investigators use what others have discovered to discover even more.
I ran across an excellent article on the Marsh Test and it’s definitely worth a read. I can imagine when this was performed in the courtroom it did elicit a few gasps.
A few useful links:
http://www.dplylemd.com/book-details/howdunnit-forensics.html
http://www.atlasobscura.com/articles/marsh-test-arsenic-poisoning
http://www.huffingtonpost.com/sandra-hempel-/arsenic-the-nearperfect-m_b_4398140.html
http://www.dartmouth.edu/~toxmetal/arsenic/history.html


Fingerprints are useful forensic science tools. They’ve been so for over 100 years. Mainly, it’s the pattern of the ridges on the fingertips that supply the useful information. We know that everyone has different fingerprints and we know that they do not change throughout the person’s life. This means that they are highly reliable sources for identification and for discrimination between two individuals. Law enforcement has employed this for years.
But several newer techniques and analyses allow investigators to go even deeper. The skin cells, that are part of a fingerprint, can often yield DNA. Chemicals in the print residue can sometimes reveal if the person has used or handled such substances as cocaine. Other analyses are underway that might make fingerprints even more useful.
One question that frequently plagues crime scene investigators is exactly when a print was laid down. This determination can make a huge difference. Let’s say that a print is discovered at a homicide scene and the primary suspect says that he had been at that location but that that had taken place a week earlier. Not on the day of the killing. Is he telling the truth? Or simply trying to throw the police off and make an excuse for the evidence they collected against him? It would be nice to know if the print was 24 hours old or seven days old.
Research is currently underway by Shin Muramoto and his colleagues and they reported their initial findings in a recent article in Analytical Chemistry. They discovered that a chemical found in fingerprints known as palmitic acid migrates away from the ridges at a predictable and consistent rate. By looking at this migration pattern they are able to determine whether the print is fresh or up to four days old. They are looking to extend this envelope to a longer period of time. But you can see, that even this level of discrimination could help—or not—- the suspect in the above scenario.
Q&A with Expanded Audio Discussions Now on the Suspense Magazine Website
Check out the new posts John Raab of Suspense Magazine and I put together. Read the Q&As and listen to the expanded discussions. Hope each proves helpful for your crime fiction.
Can DNA Be Used To Identify Multiple Assailants In a Three Decade Old Rape?
http://suspensemagazine.com/blog2/2016/12/20/d-p-lyles-forensic-file-episode-1/
In 1863, Could An Autopsy Accurately Determine the Cause of Death?
Can My Female Character Cause Her Pregnancy To Become “Stone Baby” By Shear Will?
More to come.
Want more cool questions from crime writers? Check out my three Q&A books.

More Info and List of Included Questions

More Info and List of Included Questions

More Info and List of Included Questions