From HOWDUNNIT: FORENSICS: When more than one person is found dead in a house or a car and there is no evidence of trauma, carbon monoxide toxicity is considered. The odds of two or more people dying from natural causes at the same time and the same place are extremely remote.
Such is the case of “Buckwild” star Shane Gandee who was found dead, along with two other men, in a vehicle partially submerged in mud. The supposition is that mud clogged the vehicle’s tail pipe and this allowed carbon monoxide (CO) to accumulate inside the passenger compartment, resulting is death from asphyxia.
When I first read the circumstances surrounding this tragedy, my first thought was CO. With no signs of trauma, little else made sense. Apparently the authorities have agreed that this was the cause of death.

Also from HOWDUNNIT: FORENSICS: CHAPTER 8: ASPHYXIA
CARBON MONOXIDE
Deaths from carbon monoxide poisoning are usually suicidal or accidental. It is an uncommon method for homicide, but it has been reported. Carbon monoxide is stealthy, treacherous, deadly, and common. A family is found dead and the cause is a faulty heater or fireplace. A suicide victim is found in his garage with the car engine running. Campers are found dead in a tent, a kerosene lantern burning in one corner. Each of these is due to carbon monoxide poisoning.
Carbon monoxide is a tasteless, odorless, colorless gas that is completely undetectable by humans. It results from the incomplete combustion of carbon-containing fuels—paper, wood, gasoline, and many other combustible products. Complete combustion of one of these fuels yields carbon dioxide (CO2). If there is a deficiency of oxygen or if the fi re is smoldering and doesn’t produce enough heat to drive the reaction to completion, incomplete combustion occurs and the result is the production of carbon monoxide.
Wood, coal, and gas are common carbon-containing fuels. Faulty stoves,heaters, and fireplaces, as well as the exhaust from a car engine, can fill the air with carbon monoxide. Carbon monoxide poisoning is a more common cause of death in fires than is the fire itself. Charcoal briquettes are particularly dangerous as they are designed to smolder rather than burst into flame and are also good sources for carbon monoxide. Using a charcoal grill in an enclosed space such as a garage or tent can lead to carbon monoxide buildup very quickly. Faulty butane and propane camp stoves and heaters can also be deadly.
Carbon monoxide’s treachery lies in its great affinity for hemoglobin, the oxygen-carrying molecule within our red blood cells (RBCs). When inhaled, CO binds to hemoglobin producing carboxyhemoglobin. It does so three hundred times more readily than does oxygen, and thus it displaces oxygen. The result is that the blood that leaves the lungs and heads toward the body is rich in carbon monoxide (carboxyhemoglobin) and poor in oxygen (oxyhemaglobin).
This strong affinity of hemoglobin for carbon monoxide means that very high blood levels can occur by breathing air that contains only small amounts of carbon monoxide. For example, breathing air that contains a carbon monoxide level as low as 0.2 percent may lead to blood carbon monoxide saturations greater than 60 percent after only thirty to forty-five minutes. So, a faulty heater or smoldering fi re that produces only a small amount of carbon monoxide becomes increasingly deadly with each passing minute.
This powerful attraction for hemoglobin explains how certain individuals succumb to carbon monoxide poisoning in open areas. Most people believe that carbon monoxide is only toxic if it is in an enclosed area, but this is not true. There have been cases of individuals dying while working on their cars in an open area, such as a driveway. Typically the victim is found lying near the car’s exhaust. Similarly, the newly recognized problem of carbon monoxide poisoning in swimmers and water skiers who loiter near a dive platform on the back of a powerboat with an idling engine.
The degree of exposure to carbon monoxide is typically measured by determining the percent of the hemoglobin that is carboxyhemoglobin. The signs and symptoms of carbon monoxide toxicity correlate with these levels. The normal level is 1 to 3 percent, but may be as high as 7 to 10 percent in smokers. At levels of 10 to 20 percent, headache and a poor ability to concentrate on complex tasks occur. Between 30 and 40 percent, headaches become severe and throbbing, and nausea, vomiting, faintness, and lethargy appear. Pulse and breathing rates increase noticeably. Between 40 and 60 percent the victim becomes confused, disoriented, and weak, and displays extremely poor coordination. Above 60 percent, coma and death are likely. These are general ranges since the actual effect of rising carbon monoxide levels varies from person to person. In the elderly and those with heart or lung disease, levels as low as 20 percent may be lethal. Victims of car exhaust suicide or those who die from fire in an enclosed room may reach 90 percent.
A running car engine in an enclosed garage is a common method for suicide, but it could also be used for homicide. If the killer subdues the victim by force or by way of intoxication, he could place the victim in his car and let the carbon monoxide actually do him in. When determining the manner of death, the ME looks for evidence of trauma to the victim as well as performs a toxicology screen. Finding trauma, such as evidence of a blow to the head, might change the manner of death from suicide to homicide, but finding drugs may not. Some people use multiple suicide methods to assure success and a drug overdose combined with carbon monoxide inhalation is not rare.
When more than one person is found dead in a house or a car and there is no evidence of trauma, carbon monoxide toxicity is considered. The odds of two or more people dying from natural causes at the same time and the same place are extremely remote.
Carboxyhemoglobin is bright red in color and imparts this hue to the blood. When the ME performs an autopsy and sees bright cherry-red blood, he suspects carbon monoxide poisoning as the cause of death. This finding is not absolutely conclusive since cyanide inhalation or ingestion can also result in bright cherry-red blood and tissues. Also, individuals dying from cold exposure or corpses exposed to very low temperatures may show bright red blood.
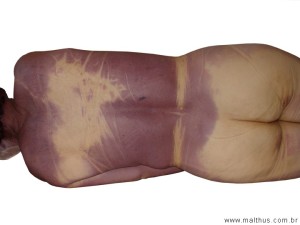
Livor mortis in these situations may also be red or pink rather than the usual blue-gray color (see Chapter Five: Time of Death, “Livor Mortis”).
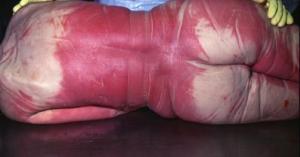
At autopsy, the internal organs in victims of carbon monoxide intoxication are also bright red. Interestingly, this color does not fade with embalming or when samples taken by the ME are fixed in formaldehyde as part of the preparation of microscopic slides. At times the presence of carbon monoxide can be found in the blood as long as six months after death.
Individuals who survive carbon monoxide intoxication may have serious long-term health problems. The brain is particularly vulnerable since it is extremely sensitive to lack of oxygen. Symptoms and signs of brain injury can begin immediately or be delayed for several days or weeks. The most common aftereffects include chronic headaches, memory loss, blindness, confusion, disorientation, poor coordination, and hallucinations. The ME may be asked to evaluate a living victim in this situation if the exposure was due to a criminal act or if a civil lawsuit is involved.






































